 This chemistry video tutorial explains how to solve combined gas law and ideal gas law problems. It covers topics such as gas density, molar mass, mole fraction, dalton's law of partial pressure, and graham's law of effusion. This video contains plenty of examples and practice problems.
This chemistry video tutorial explains how to solve combined gas law and ideal gas law problems. It covers topics such as gas density, molar mass, mole fraction, dalton's law of partial pressure, and graham's law of effusion. This video contains plenty of examples and practice problems.
Here is a list of topics:
1. Pressure - Force over Area - 1 Pascal = 1 Newton per Square Meter
2. Pressure & Number of Collisions By Gas Molecules
3. Pressure Unit Conversion - atm, torr, mm hg, pa, and kpa
4. Combined Gas Law Formula
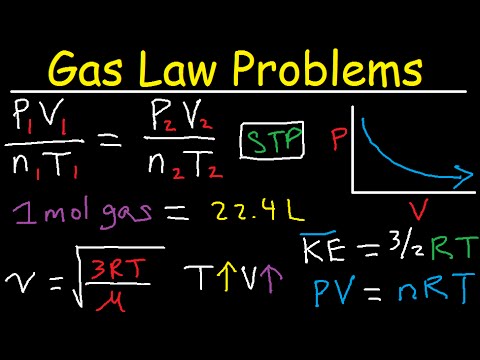
5. Boyle's Law - Pressure vs Volume - Inverse Graphical Relationship - P1V1=P2V2
6. Charles Law - Volume vs Temperature - Directly Proportional Linear Relationship - V1/T1=V2/T2
7. Gay Lussac Law - Pressure vs Temperature - Direct Variation P1/T1=P2/T2
8. Avogadro's Law - moles & Volume - Graph V1/n1=V2/n2
9. The effect of Volume on Pressure - Molecular Collisions
10. Average Kinetic Energy of a Gas Equation - KE=3/2RT
11. Temperature, Average KE, Molecular Speed
12. Practical Application of Gases - Balloon Expansion
13. Why Do Helium Filled Balloons Rise - Gas Density & Molar Mass
14. Pressure vs Elevation / Altitude - Pressure at Sea Level - 1 atm
15. Internal vs External Forces - Pressure
16. Combined Gas Law Word Problems
17. Temperature Unit Conversion - Celsius to Kelvin
18. Ideal Gas Law Problems - PV=nRT
19. Gas Constant R = 0.08206 L atm mol^-1 K^-1
20. Units of Pressure, Volume, and Temperature
21. milliliter to Liter Metric System Conversion - mL to L
22. Gas Density Problems at STP
23. Standard Temperature and Pressure - 0K and 1 atm
24. Molar Volume at STP - 1 mole of gas = 22.4 Liters
25. How to identify the unknown gas by calculating the molar mass
26. Relationship between gas density, molar mass, pressure, and temperature for rigid container or expandable balloon
27. Mole Fraction of Nitrogen (N2), Oxygen (O2) and Argon (Ar) gas
28. Partial Pressure and Mole Fraction Practice Problems
29. Dalton's Law of Partial Pressure
30. Mole Fraction as a Percentage
31. Gas Stoichiometry Problems - Mass to Mass and Volume to Volume Conversions
32. How to calculate the mass of hydrogen gas (H2) collected over water using the vapor pressure of water and the partial pressure formula
33. Total Pressure, Vapor Pressure, & Partial Pressure
34. How To Identify the Unknown Gas Using Collected Over Water Problems
35. Root Mean Square Velocity Formula / Equation Derivation
36. Graham's law of Effusion Formula
37. Effusion vs Diffusion Concepts
38. How To Calculate the Rate of Effusion and Identify the Unknown Gas
39. Pressure vs Molar Mass & Molecular Weight vs Speed / Velocity
40. Kinetic molecular Theory of Ideal Gases
41. Attractive & Repulsion Forces, Elastic Molecular Collisions, Negligible Volume, and Average Kinetic Energy & Temperature
42. Real Gases vs Ideal Gases - High Temperature & Low Pressure
43. Phase Diagrams - Vaporization, Evaporation, Sublimation and Deposition
Gas Law Problems Combined & Ideal - Density, Molar Mass, Mole Fraction, Partial Pressure, Effusion diffusion in plants | |
| 2,112 Likes | 2,112 Dislikes |
| 179,291 views views | 1.16M followers |
| Education | Upload TimePublished on 16 Sep 2016 |
No comments:
Post a Comment